Placing a needle-based implant in a mouse
The mouse is anesthetized by 1.5% - 2% of isoflurane in oxygen or i.p. injection of ketamine/xylazine and placed into a silicon rubber holder.

The skin is shaved and cut on the mouse head, and an etchant is applied to the skull for 30 to 60 seconds. After the etchant is washed with sterile water, the skull is briefly dried, and a thin layer of Optibond is applied on the bone. Optibond is cured by blue light for about 10 seconds.
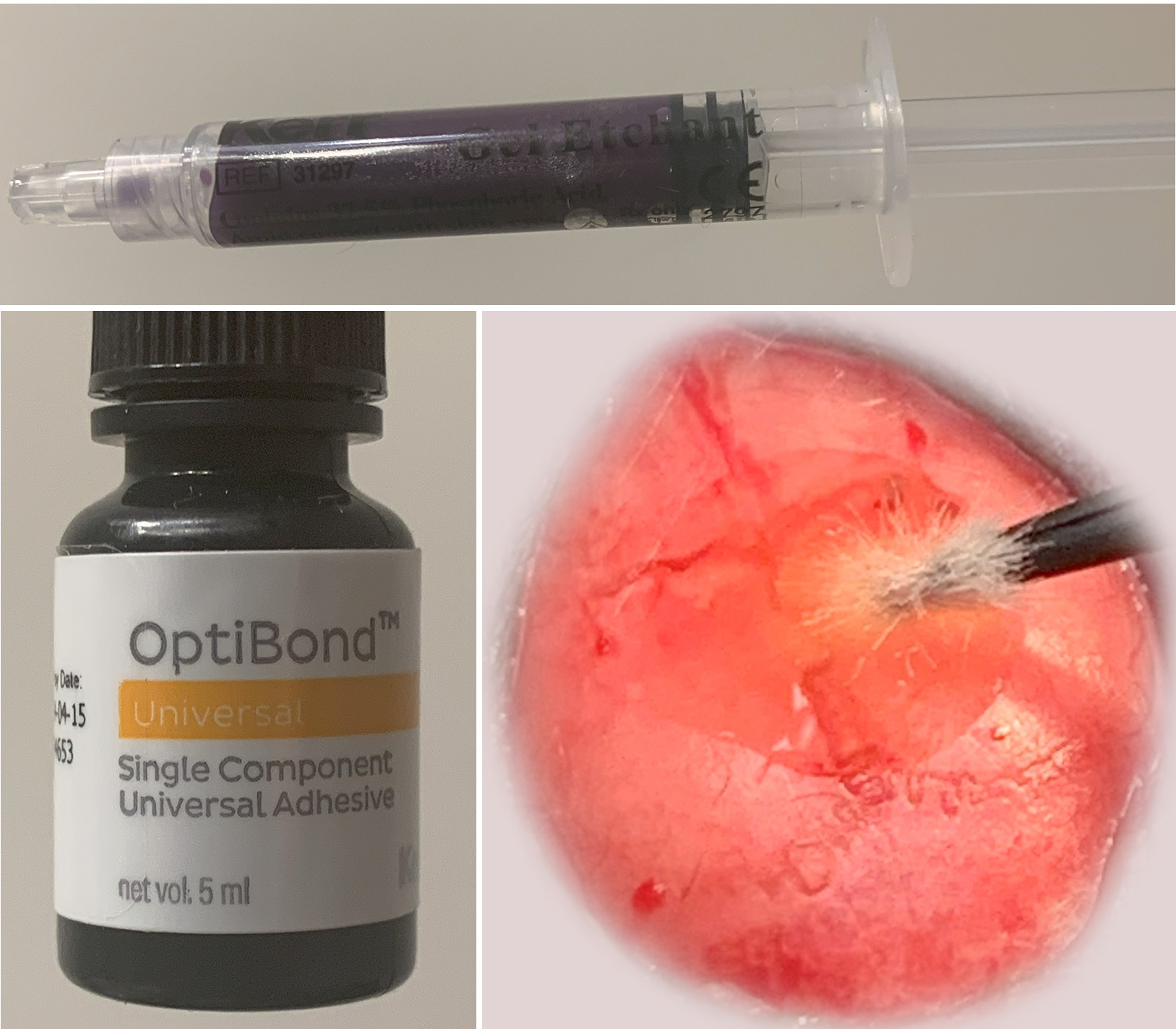
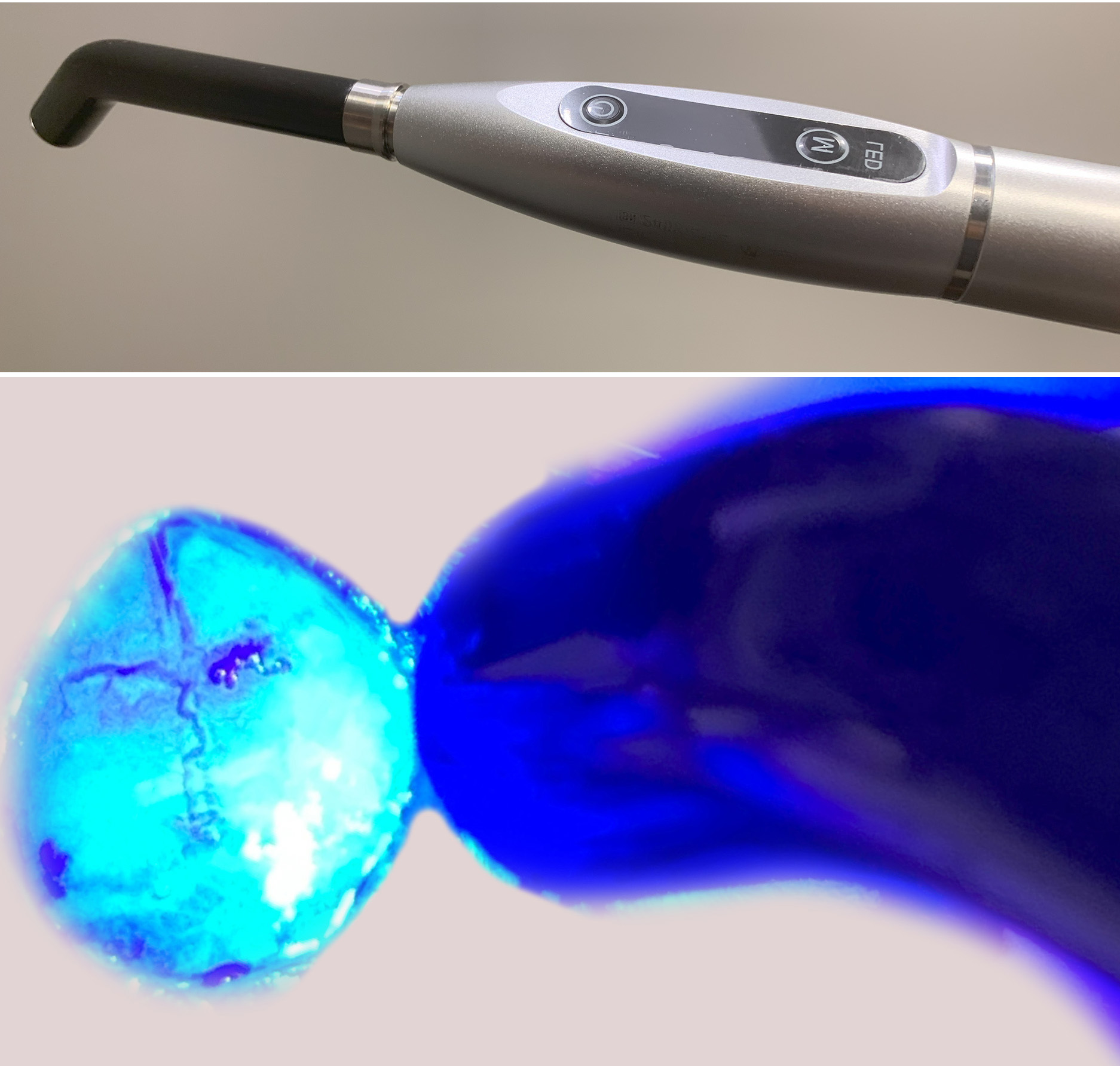
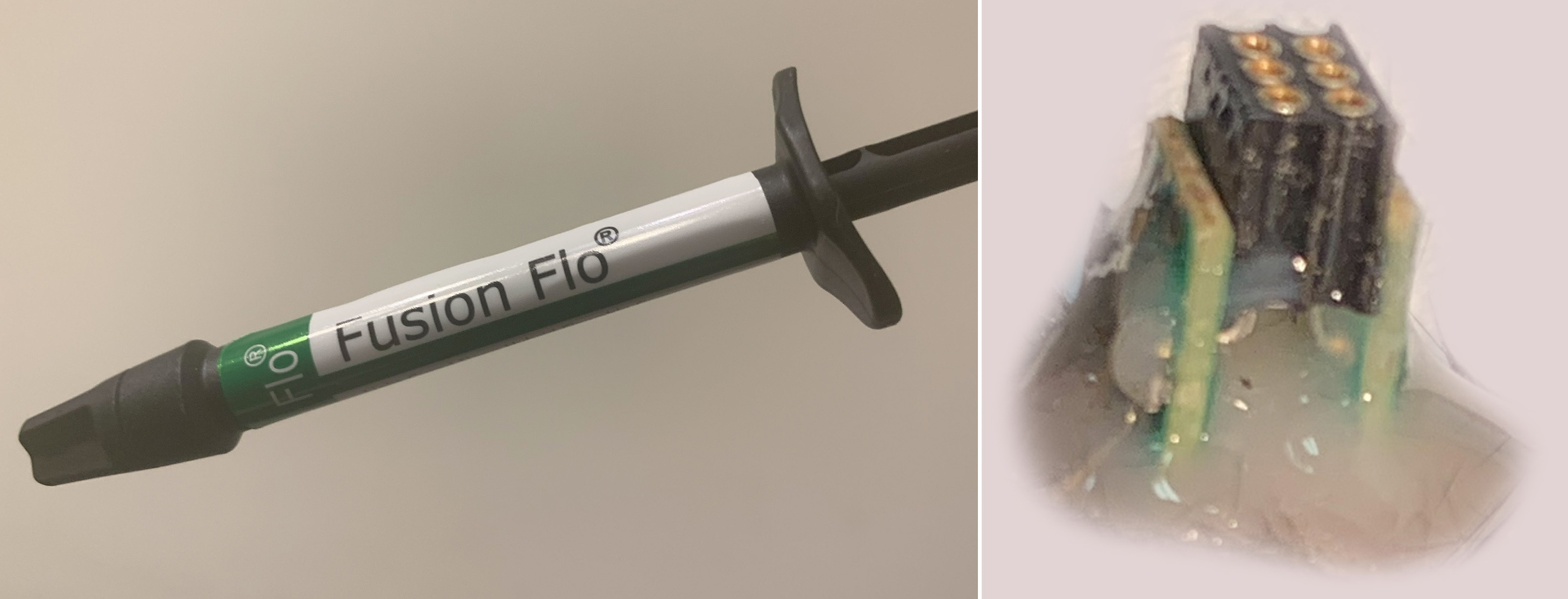
The implant is gently pressed into the skull. It is then glued to the skull using a light-curable composite (e.g. Fusion Flo). EMG electrodes are inserted under the skin onto the nuchal muscles. All implant components are further fixed to the skull using additional light-curable composite.
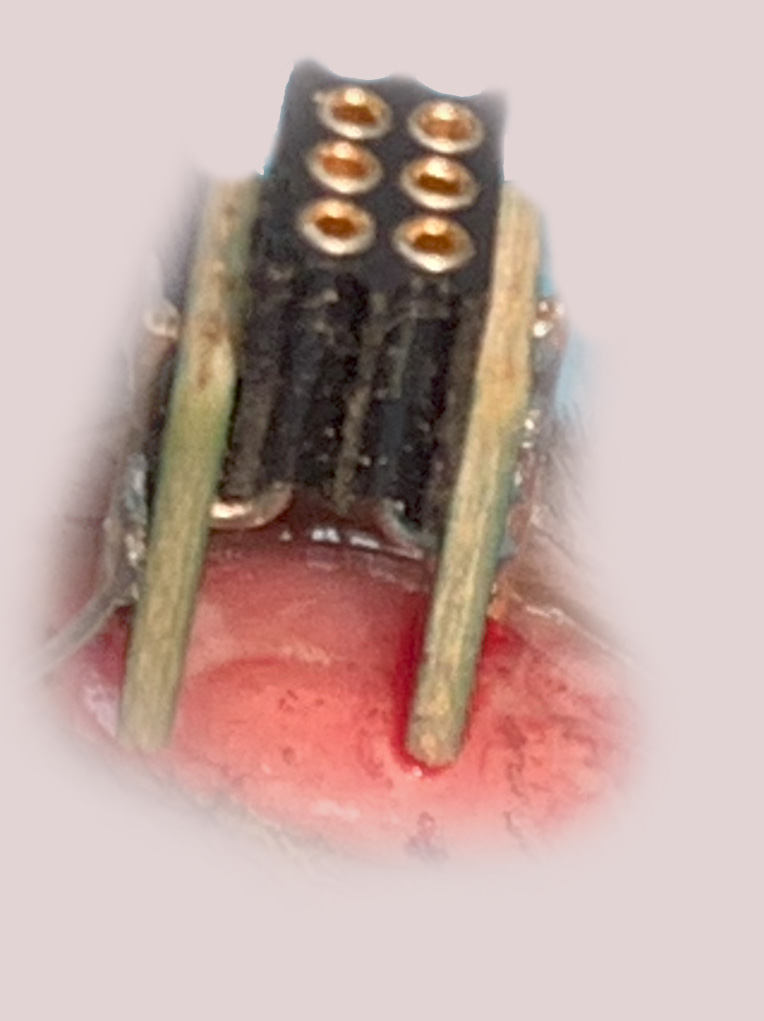
Connecting a telemetry transmitter to the animal
A telemetry transmitter and batteries are placed in the container and attached to the cable. Movement of the mouse is not substantially reduced even if the total weight of the container exceeds an ounce (28 g).
The transmitter can be powered by a battery located outside of the cage by connecting it via a slip ring.

The container-cable assembly is attached to the animal and then hanged on the aluminum rail of the cage insert, so that the container can slide and rotate, allowing maximum mobility of the animal.

